Manipulated units of a combined test for COVID-19 and influenza have reached the European market. The manufacturer has confirmed that batch FCO24090516 is fake.
The Spanish Agency for Medicines and Health Products (Agencia Española de Medicamentos y Productos Sanitarios) has been alerted by the Portuguese authorities about the circulation of counterfeit self-testing kits combining coronavirus and influenza A+B detection.
The original product was manufactured by Safecare Biotech, based in Hangzhou (China), but the detected units carry the batch number FCO24090516, which the company has declared as counterfeit.
Although the AEMPS has launched an investigation to trace distribution in Spain, so far there is no evidence that these manipulated units have reached pharmacies or distributors in the country.
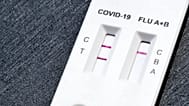
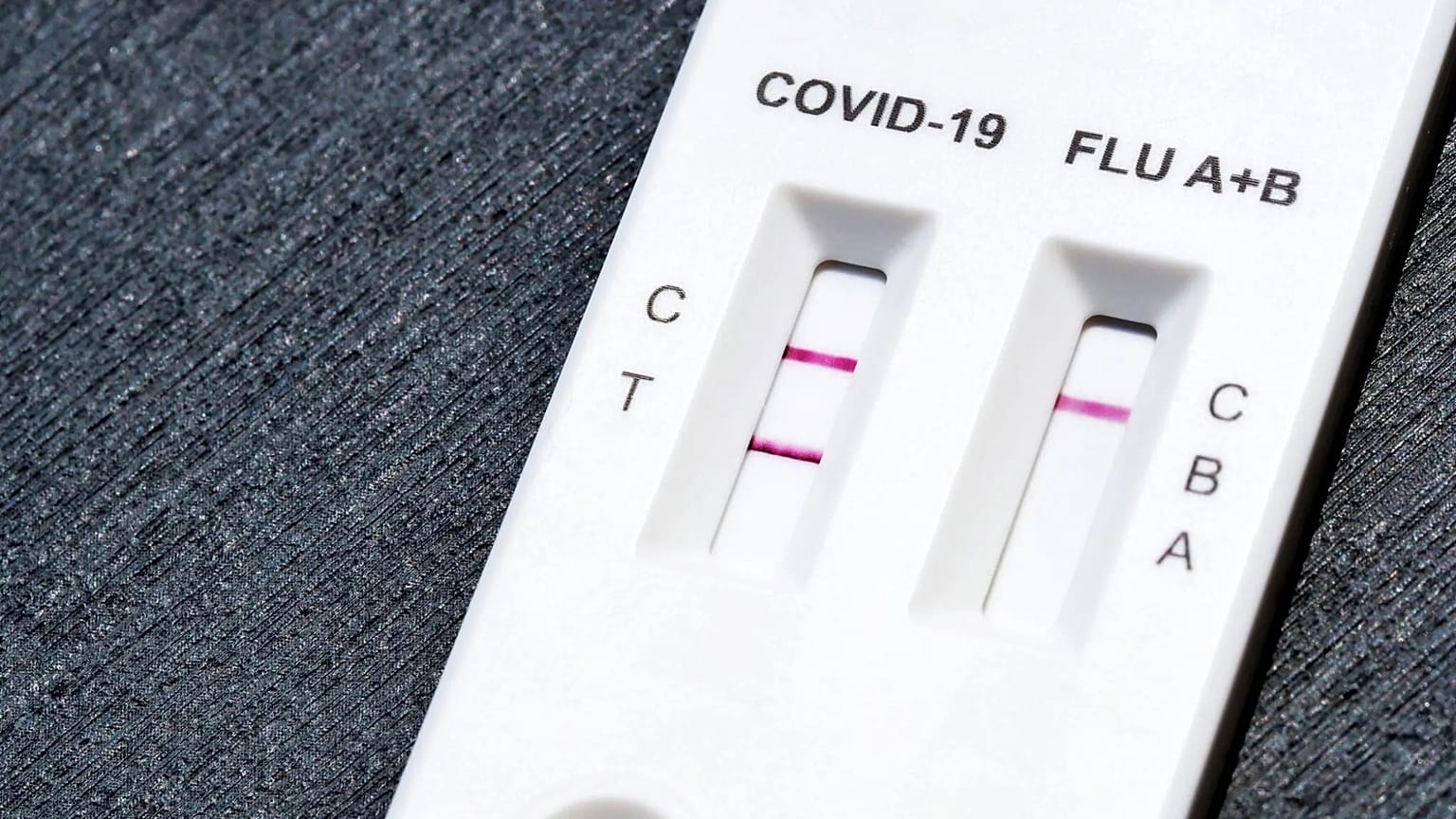
There are legitimate versions of the same test with the same reference (FCO-6032), which complicates the immediate visual identification. Here you can see an image of the test, in which the external appearance is the same except for a small detail.
How to identify manipulated products
The counterfeit tests have three obvious alterations. First, they have a label affixed with modified information: batch FCO24090516, date of manufacture September 2024 and expiry date September 2026.
Second, the test cassette and the tube with the extracting solution are lacking the lot number and dates. Third, the swab is not that of the legitimate manufacturer, which works with Dalian Rongbang Medical (Lotus NL is its European representative).
Authorities insist that these products should only be purchased from pharmacies. Otherwise, there are no guarantees about origin, storage conditions or professional advice. If anyone has such a test at home, the advice is clear: do not use it.
The adulteration of medical devices is not a minor problem. These devices are designed to make decisions about personal and collective health, and an erroneous result can lead to undetected infections or unnecessary treatment.