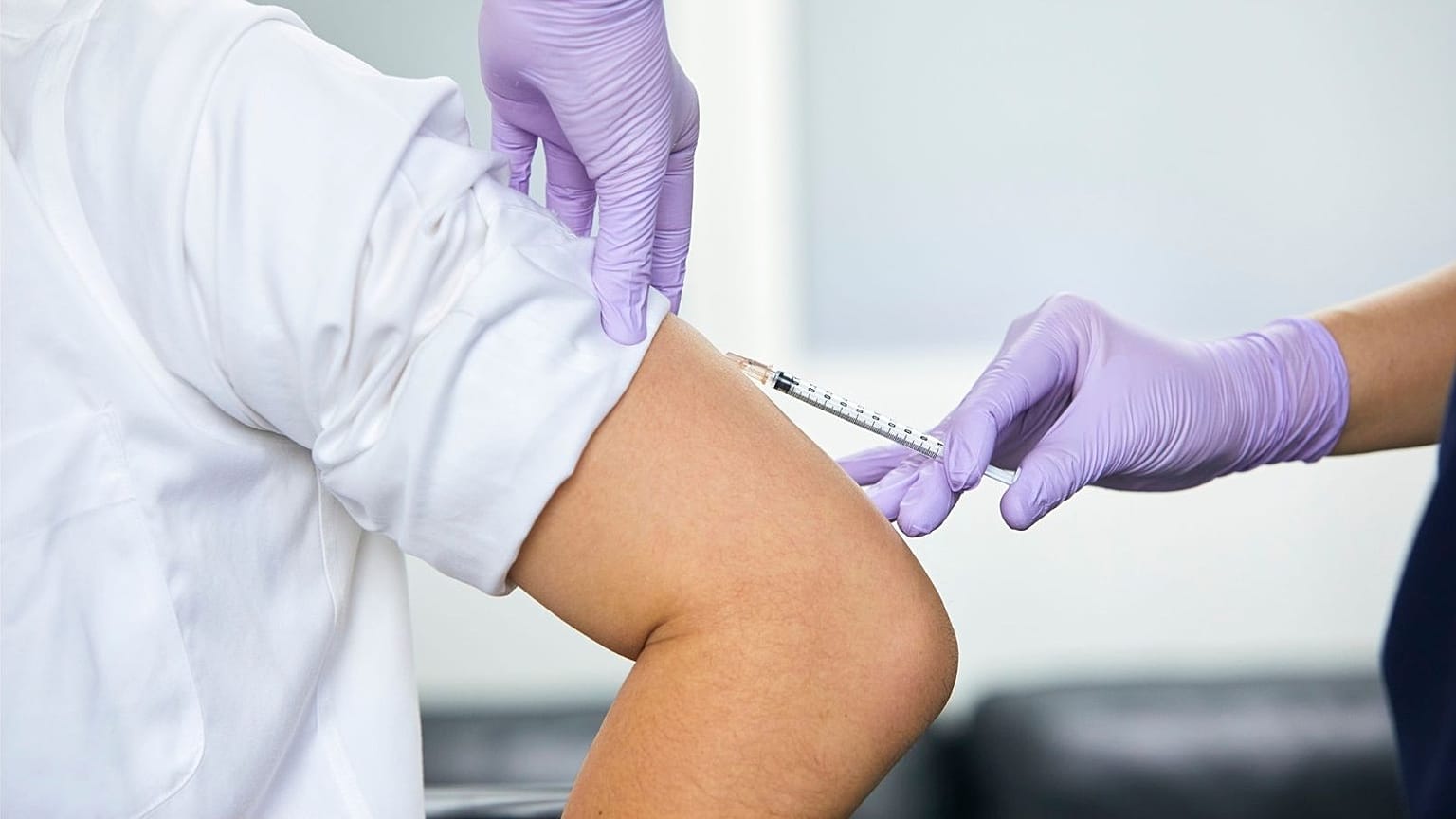
The drug, known as cabotegravir, must be administered every two months.
People in England and Wales will soon have access to a long-lasting HIV prevention jab for the first time.
 ADVERTISEMENT
ADVERTISEMENT
 ADVERTISEMENT
ADVERTISEMENT
The National Institute for Health and Care Excellence, which issues guidance on new drugs, recommended the jab for adults and young people who are at high risk of acquiring HIV but cannot take the medicine in pill form.
The drug, known as cabotegravir, must be administered every two months.
It is a form of pre-exposure prophylaxis (PrEP), which works by preventing the virus from replicating and spreading within the body. It reduces the risk of acquiring HIV among both adults and adolescents.
In 2024, more than 111,000 people in England accessed PrEP through sexual health clinics, according to the United Kingdom’s Health Security Agency (UKHSA).
NICE estimates that up to 1,000 people annually will benefit from cabotegravir’s availability through the National Health Service (NHS) in England. It is expected to roll out in the coming months.
“Today's recommendation for cabotegravir marks a significant milestone – this is the first injectable HIV prevention treatment that is available to patients,” Helen Knight, NICE’s director of medicines evaluation, said in a statement.
Additional HIV prevention drugs on the horizon
Notably, cabotegravir is different from another HIV prevention jab, called lenacapavir,that has been hailed as a major medical breakthrough because it was highly effective in clinical studies and must only be administered twice per year.
Regulators in the European Union and United States approved lenacapavir earlier this year, which could speed UK regulators’ decision once the drugmaker, Gilead, seeks approval there.
In wealthy countries, a yearly course of lenacapavir is priced at more than $28,000 (about €24,000) per person, according to the medical access group Unitaid.
Last month, Unitaidannounced plans to make a cheaper, generic version of the lenacapavir jab available in 120 lower income countries beginning in 2027.