A TikTok weight loss trend has caused shortages of Ozempic. What are the risks for those using this diabetes drug off-label?
A diabetes drug is facing shortages worldwide and causing health concerns as users flood social media with posts boasting its properties as a "wonder" weight loss hack.
 ADVERTISEMENT
ADVERTISEMENT
 ADVERTISEMENT
ADVERTISEMENT
France's drug safety agency said this week it was ramping up surveillance measures to ensure that prescriptions of the treatment, Ozempic, are limited to patients with type 2 diabetes.
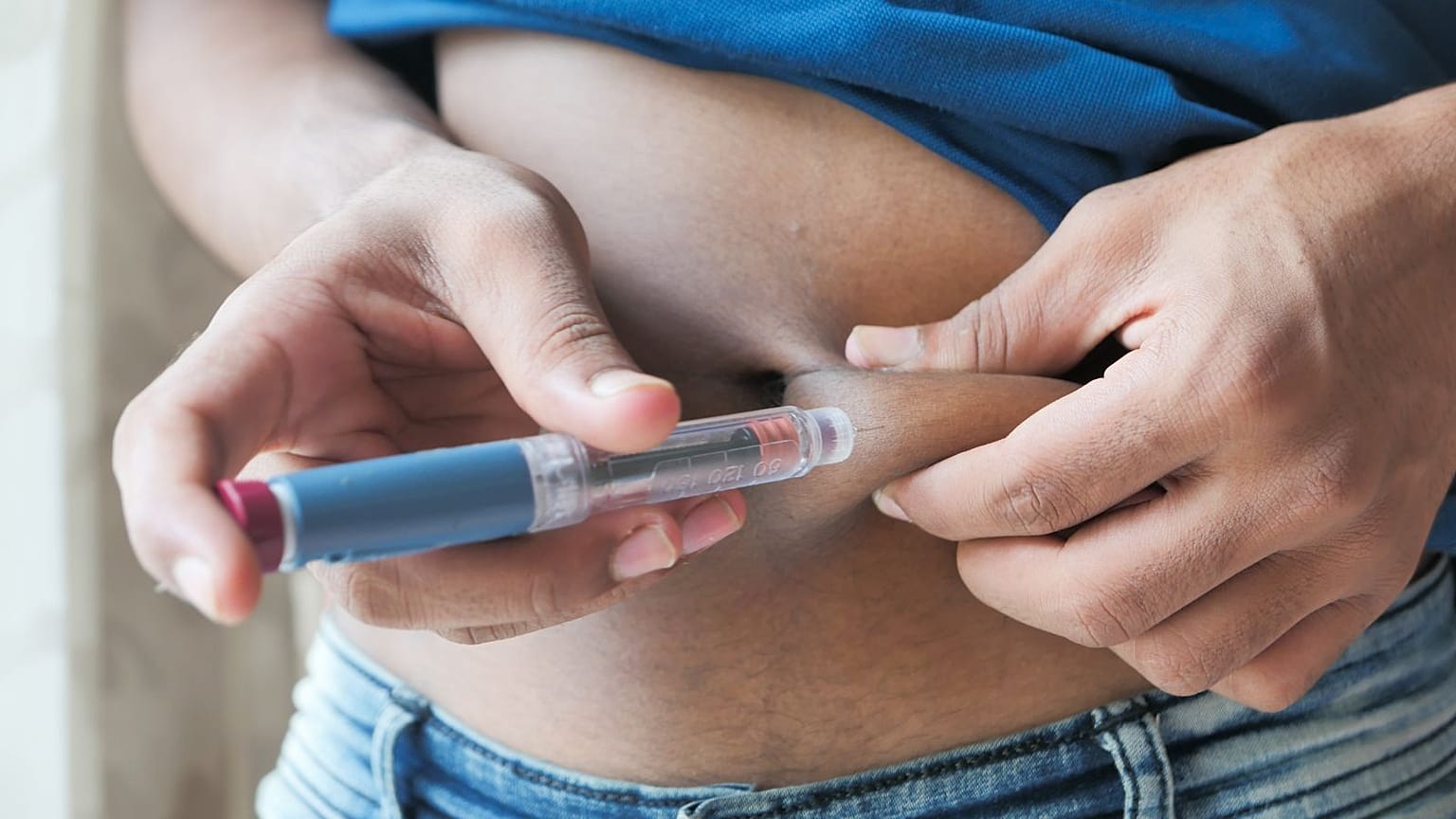
Produced by the Danish company Novo Nordisk, Ozempic is an injectable drug that regulates blood sugar levels and insulin. It’s usually prescribed to adults suffering from Type 2 diabetes, the most common type of diabetes.
But the drug’s active ingredient, semaglutide, also mimics a hormone called glucagon-like peptide-1 (GLP-1) that targets areas of the brain that regulate appetite and food. Because it makes users feel full faster, it can lead them to lose weight.
As a result, Ozempic has been touted on the Internet as a miracle diet drug. On TikTok, the hashtag #Ozempic already has over 600 million views and counting.
Even Twitter's new boss, the billionaire Elon Musk, has credited Ozempic - alongside a similar drug, Wegovy - as one of the reasons he shed 13 kg.
On Facebook, groups dedicated to the use of Ozempic for weight loss have tens of thousands of followers.
The global spike in demand has already caused a shortage of Ozempic in Australia that is expected to continue to disrupt supply for another "few months".
Australian authorities have called on health professionals to avoid initiating new prescriptions of Ozempic and, when treating patients with obesity, to consider alternatives to semaglutide "until supply stabilises".
France's national drug safety agency issued similar instructions in September when it urged doctors to only initiate new GLP-1 drug prescriptions for patients with Type 2 diabetes with a history of stroke or heart disease.
On Wednesday, the regulator said recent estimates suggested that over the course of a year, some 2,185 patients in France were given Ozempic even as they were not diabetic. That means around 1 per cent of Ozempic prescriptions covered by state health insurance were "misused," it said.
The agency stressed that these off-label prescriptions were squeezing out diabetic patients who really needed the drug, and it warned of "potentially serious" side effects such as gastrointestinal disorders, pancreatitis and low blood sugar.
Authorities in the UK and Australia have also issued warnings to influencers promoting these drugs online. In France, pharmaceutical advertising to the general public is a highly regulated practice, which requires prior authorisation and is reserved for medicines that are not reimbursed by the public health insurance system.
In an emailed statement, Novo Nordisk said it "does not promote or endorse off-label use" of its products and that it collaborates closely with health authorities to ensure patient safety.
The drumaker said in December that it was taking the shortages seriously and had invested $1.6 billion (€1.5 billion) in 2022 alone to expand its production capacity and catch up with "stronger than anticipated" demand.
What are the health risks?
The shortage has consequences not only for patients with diabetes who need to follow their treatment on a daily basis but also for the people who are turning to these drugs exclusively for weight loss.
"It can be mild digestive side effects, sometimes severe constipation and inflammation of the pancreas. In rare cases, this can lead to cancer," François Montastruc, a pharmacologist at the Toulouse University Hospital (Haute-Garonne), told the French TV channel France 3 late last year.
The instructions for Ozempic published by the European Medicines Agency state that nausea, diarrhoea and hypoglycaemia are "very common" side effects. Like all medicines, Ozempic also has contraindications that a doctor should take into account when prescribing it.
Prescription guidelines vary from country to country, but Ozempic has not been extensively studied for use in patients without diabetes or excess weight.
The rampant off-label use of a diabetes drug brings back memories of the Mediator scandal in France. The drug was widely prescribed as an appetite suppressant and for weight loss until it was linked to fatal heart problems and pulled from the French market in 2009.
Ozempic was not licensed for weight loss, but in 2021 the FDA approved semaglutide, its active ingredient, under the brand name Wegovy for chronic weight management in adults with obesity or overweight with at least one related condition - such as high blood pressure, Type 2 diabetes, or high cholesterol.
The US marketing authorisation specifies that Wegovy carries warnings for “inflammation of the pancreas (pancreatitis), gallbladder problems (including gallstones), low blood sugar, acute kidney injury, diabetic retinopathy (damage to the eye's retina), increased heart rate and suicidal behavior or thinking”.
Regarding Ozempic, the US National Library of Medicine warns that the injectable drug “may increase the risk that you will develop tumours of the thyroid gland, including medullary thyroid carcinoma (MTC) which is a type of thyroid cancer. Laboratory animals who were given semaglutide developed tumours, but it is not known if this medication increases the risk of tumours in humans”.
Novo Nordisk said it was up to doctors to choose the best treatment approach for their patients, and that the drugmaker's GLP-1 medicines had been on the market for more than 10 years.
"To date, the safety data from trials and post-marketing safety surveillance have not identified any risks that outweigh the benefit of treatment," the company said.
A long list of diverted drugs
It’s not the first time that a drug has been diverted from its intended purpose to be used for aesthetic reasons.
In 2021, a BBC documentary warned about the dangerous use of the medicated syrup apetamin by female influencers, this time to gain weight quickly and achieve an extreme hourglass figure. The drug, which is not approved in France or the UK, can cause drowsiness and in severe cases can lead to liver failure or even comas.
A number of beauty “tricks” involving unlicensed drugs or off-label uses have come under fire in recent years. In a 2017 article on The Conversation, two French researchers warned about the use of Homeoplasmine, a medicated ointment containing boric acid that beauty pundits have touted as a moisturising balm, particularly for the lips.
Using anti-haemorrhoid creams to supposedly reduce puffiness and bags under the eyes has also made headlines. These creams typically contain hydrocortisone and are not suited for the thin, fragile skin around the eyes.
In general, health authorities recommend that products from the medicine cabinet should be used with caution and under the advice of a health professional.