
Disrupting Itself: How Philip Morris International is Quitting Cigarettes
*Disclaimer: The views expressed by people interviewed for this article are their own.
A new vision
A decade ago, the world’s most successful tobacco company, Philip Morris International (PMI) began the process of upending its lucrative and longstanding cigarette manufacturing business. The former CEO and Executive Chairman of the Philip Morris board, André Calantzopoulos made a bold commitment to develop less harmful alternatives for its millions of adult consumers, for global public health and the future of the business.
It’s no secret smoking cigarettes has a detrimental effect on human health. Tobacco companies that are not innovating or taking measures to develop better alternatives for adult smokers - who would otherwise continue smoking - potentially face operating risks as seen in other industries such as fossil fuels. Recently UK’s Prime Minister, Boris Johnson announced plans to phase out the sale of petrol and diesel vehicles by 2035 to reduce air pollution in England. This is an example of growing regulation and pressure from stakeholders to get the private sector to accelerate transformation and find new, better ways. Whilst restrictions on cigarette sales & communications to adults are increasing for the tobacco industry, PMI has taken a leadership position to proactively transform itself from the inside and make drastic changes to its business.
Now, after a decade of internal research and action, the tobacco multinational is embarking on a global communications effort to help explain the science behind alternative products with policy makers, health bodies and the one billion smokers globally.

Where did it begin?
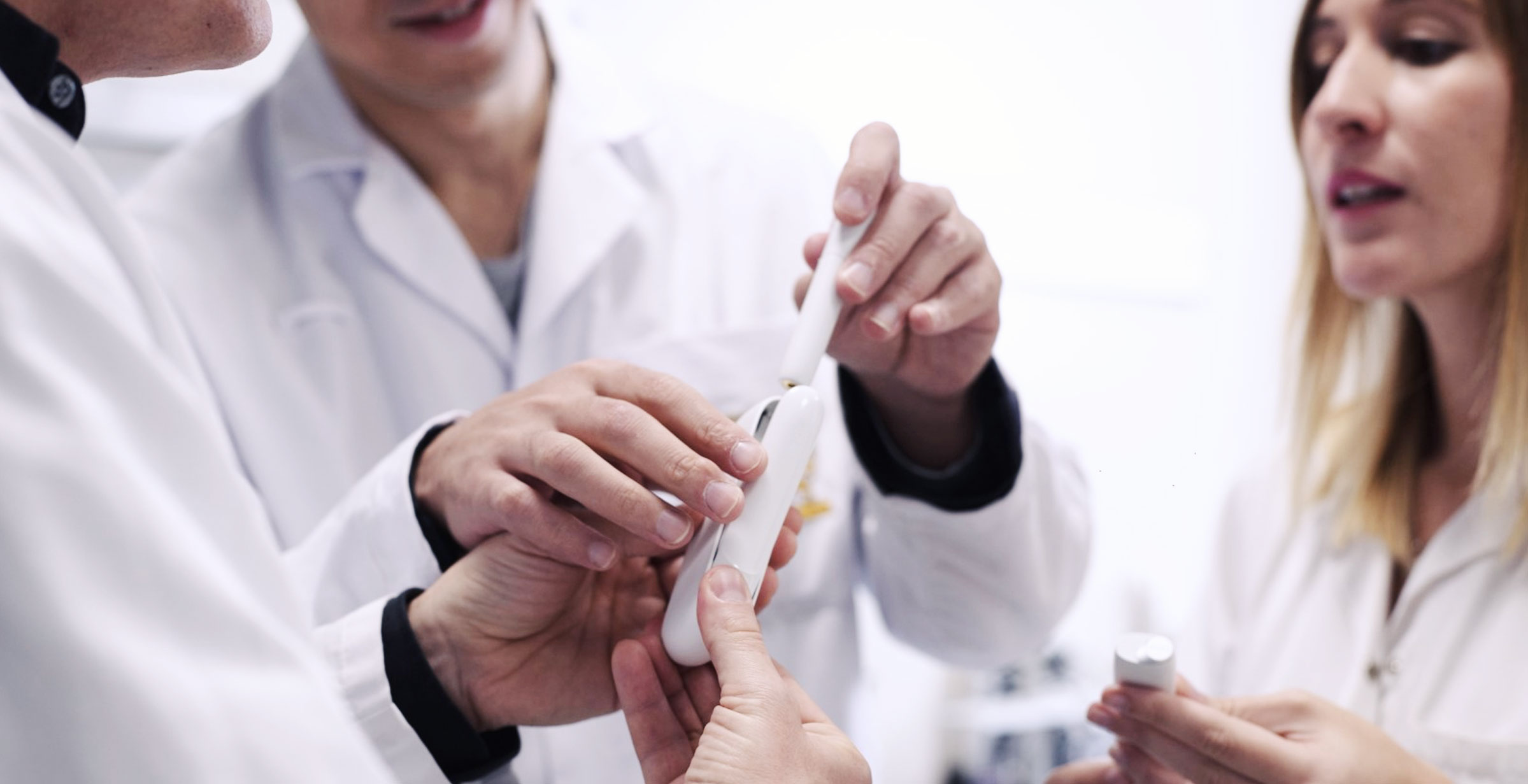
Since the CEO declared the company’s ‘smoke free vision’, PMI has invested over USD$ 8.1 billion to build research and development capabilities to accommodate laboratory studies and clinical trials of new products, such as the electrical heated tobacco system called IQOS. The company has employed more than 400 scientists and engineers in its Swiss-based R&D center to lead the transformation and build the intellectual credibility to prove to regulators and its customers that it can be part of the solution.
Innovation didn’t stop there. PMI has also engaged with Switzerland’s rich startup ecosystem partnering with the MassChallenge incubator which connects entrepreneurs developing new technologies together with corporates looking to leverage outside ideas. Matt Lashmar, Managing Director of the MassChallenge in Switzerland has been working with PMI as a corporate partner for some time.
“We’ve been able to work with PMI on a variety of areas relating to product innovation. Not only in technological solutions to develop the smokeless products but also on other digital elements such as packaging. PMI is taking a leadership position to try to reduce the smoking epidemic, we’re pleased to support that cause”, Mr Lashmar said.
How does it work?
PMI’s leading smoke-free products work by heating tobacco electronically, rather than burning it. The heating of tobacco emits significantly less harmful chemicals than when tobacco is burned. So far, 17.5 million adult smokers, who would otherwise have continued to smoke cigarettes, have opted for a better choice and use PMI’s smoke-free product. However there is still a long way to go and PMI believes that more can be done if the tobacco industry is able to talk to adult smokers and inform them about better options and the science behind it.
“The uptake of these products is higher where there is an environment that allows better tobacco alternatives to be seen and talked about as replacements. We need to phase out combustible tobacco products as soon as possible”, said Mr Calantzopoulos.
FDA Authorisation
PMI has had some positive news which may pave the way for tobacco regulators globally. In 2020, the U.S. Food and Drug Administration (FDA) authorised the marketing of PMI’s IQOS as a modified risk tobacco product (MRTP). In doing this, the FDA was satisfied that research studies showed that switching completely from conventional cigarettes to IQOS significantly reduces exposure to harmful or potentially harmful chemicals. This decision follows a review of the extensive scientific evidence the company submitted to the FDA in 2016.
This is the second set of nicotine products ever to receive such authorisation from the FDA (after Swedish Snus) and now means the company can market - to existing adult smokers – its electronically heated tobacco system as a modified risk tobacco product within the US. It is a landmark decision and potentially paves the way for governments to regulate alternative products to differentiate them against combustible cigarettes.
“The FDA’s decision provides an important example of how governments and public health organizations can regulate smoke-free alternatives to differentiate them from cigarettes in order to promote public health. The best choice for health is to never start smoking or to quit altogether. For those who don’t quit, the best thing they can do is switch to a scientifically substantiated smoke-free product. ” argued Mr Calantzopoulos.
Beyond the US
Despite the FDA decision, the industry still faces a long road ahead as tobacco marketing is highly regulated and many governments are reluctant to change policies on any tobacco products regardless of the different risk profiles.
Shane MacGuill, Senior Head of Tobacco Research at Euromonitor International welcomes the innovation in the industry and believes the IQOS products could be a bridging product to even better alternatives as the industry evolves over the decades. He argues that to move forward, there needs to be not only an emphasis on data from modified risk tobacco products but also an open dialogue about nicotine, the addictive substance in tobacco products.
“Understanding the different substances in tobacco products as well as the root causes of smoking is as important as the health data on e-cigarettes and vapor products. Whilst we should continue to have a healthy skepticism towards tobacco companies, I think stakeholders including the media can do a better job at portraying the different sides of the debate. Having an open and factual conversation about the evolving industry is really important”, Mr MacGuill said.
Whether these alternative products completely phase out the infamous cigarette remains to be seen. What we do know is how important science and facts are to drive decisions and government policies especially when it’s related to health. Debate and science are leading to innovation-driven progress in other industries, why is the tobacco industry any different?
More about Philip Morris’ transformation and development of better alternatives





