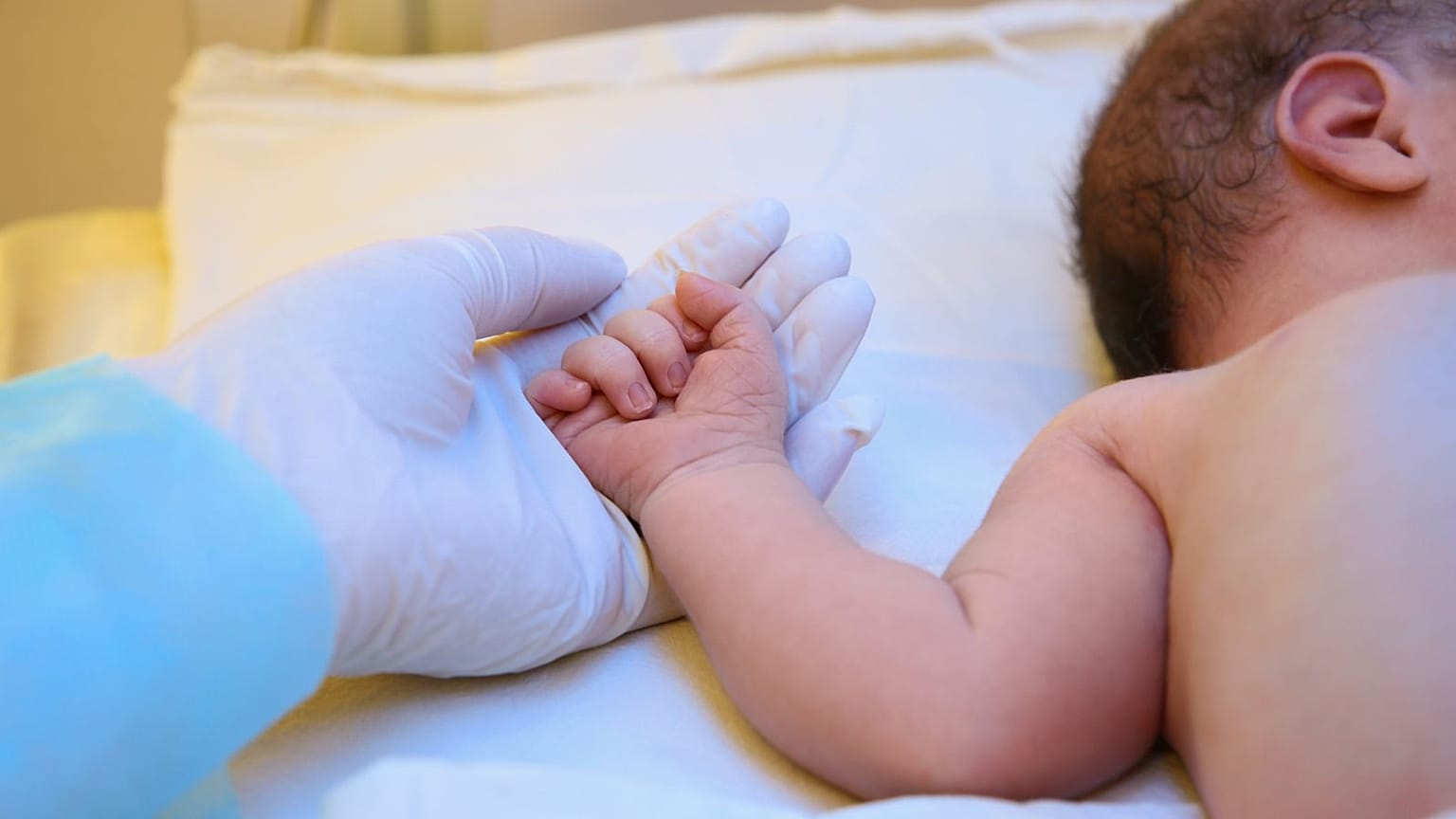
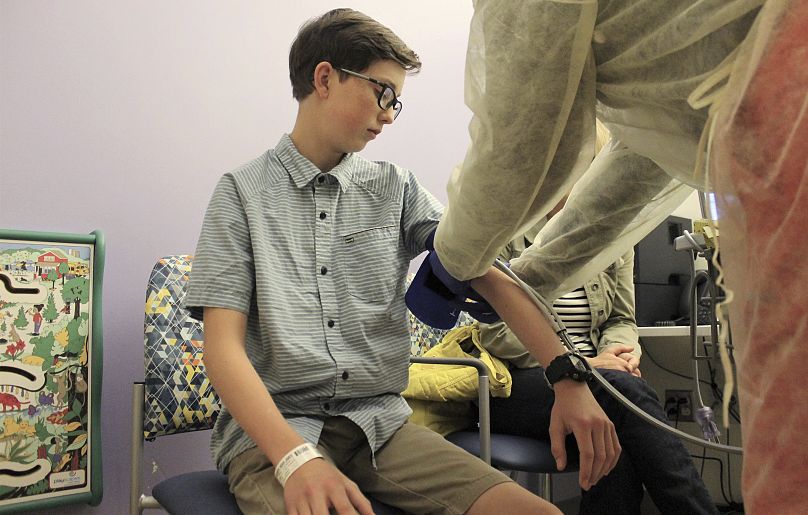Children are suffering because of a lack of approved medication, but testing on minors continues to pose a number of challenges.
Segolene Gaillard, a clinical project leader in Lyon, France, explains that not everyone understands medical research.
 ADVERTISEMENT
ADVERTISEMENT
 ADVERTISEMENT
ADVERTISEMENT
“When I tell people what I do, some say: ‘Really? You carry out clinical trials with children? That’s unethical.’ I tell them it would be even more unethical to prescribe a drug to a child without knowing if it’s truly effective and safe.”
Since 2007, Ms Gaillard has been helping doctors organise research with minors, aiming to make clinical trials more adapted to children’s needs.
With this motive in mind, she coordinates the national project ‘Kids France’, within the Hospices Civils de Lyon, an initiative that brings young people together to discuss and review paediatric research projects.
“It's necessary to ask young people and children what they need and want if we are to carry out high-quality, feasible paediatric clinical trials,” she says.
“We do this a lot for adults but we haven’t been doing it enough for children.”
A stubborn problem
According to a report published by the European Commission earlier this year, children’s medical needs aren’t being “sufficiently met” because too few treatments are being developed for the paediatric population.
Whilst this may be a tragic statement, experts say it's not a new or unexpected one.
Since the turn of the century, the EU has been working on initiatives to boost research into treatments for children.
This area of medicine hasn’t typically been a priority for pharmaceutical companies, although some progress has been made in the last twenty years.
In part thanks to EU legislation, the number of paediatric clinical trials in EudraCT, a European database, increased by 50 % between 2007 to 2016, rising from 8.25 % to 12.4%.
One of the key turning points was in 2006, when Paediatric Investigation Plans (PIPs) were introduced.
In simple terms, a PIP is an outline of how a treatment will be tested on children, and before putting an adult medication on the market, companies must now submit one of these plans and have it approved.
Exceptions can be made if the new product is known to have no paediatric benefit for the target illness.
Yet whilst this rule aims to support advancements in children’s medicine, it doesn’t always work smoothly, as plans are sometimes submitted and then postponed for several years.
Currently, 86% of PIPs include deferrals, meaning that the completion of these trials can be delayed until after new treatments are given market authorisation.
The European Medicines Agency (EMA) recently estimated that the average expected PIP duration was 9.18 years.
And it's not just the completion of the trials that's a problem, but also the submission of the PIPs in the first place.
The EU requires drug companies to submit a PIP - or a waiver application - no later than completing a so-called PK study with adults.
A PK test looks at how an adult’s body will affect the new drug.
But despite this requirement, the EMA published a report in 2021 showing that a number of PIPs and waivers weren’t being submitted on time.
To add to this, the EU is concerned that waivers are being granted in inappropriate cases.
At the moment, a company isn’t required to submit a PIP if they are manufacturing a drug to treat an illness that doesn’t exist in children.
However, just because the drug can’t be used in the same way, this doesn’t mean it won’t be effective in treating similar diseases that do exist in the paediatric population.
EMA data shows that in the last five years, 60% of new drug applications were obliged to carry out PIPs and 40% were exempt.
The ethics of consent and assent
Funding medicines for children seems to be a pretty uncontroversial goal, but the practical and ethical hurdles continue to stall progress.
“In the 1970s, some voices were saying that we should never perform any clinical trials with children,” explains Marcin Waligóra, a professor and vice dean at Jagiellonian University Medical College in Poland.
“Basically because children are not able to give informed consent, [some ethicists thought] we somehow would exploit them in clinical trials," he says.
As time has passed, the medical community has warmed to clinical testing with minors, because ultimately a lack of testing means a lack of safe treatments.
That said, this doesn’t mean that consent isn’t still a thorny issue.
Before enrolling in a medical trial, adult participants must confirm that they accept the potential risks of the study after receiving all of the relevant information.
For children, this becomes trickier, as it is harder to determine whether the child can fully understand their decision.
Added to this, some trials are conducted on infants who are still non-verbal, meaning it's impossible for them to express an opinion.
Different countries set different age thresholds for medical consent, but in Poland, where Marcin Waligóra is based, the legal age is set at 13 years old.
Until minors reach 18, they still need to have the agreement of a parent or guardian to take part in a trial, but the Polish law nonetheless recognises a teenager’s ability to consent.
Before this age, Professor Waligóra points to a non-legal concept called ‘assent’, sometimes referred to as an ‘opinion’.
He says that even if a minor is too young to fully understand the trial, researchers should “include the child in the decision-making process as fast as possible”. In order to do this, they need to think about the best way to explain their work and how to design their trials in a child-friendly way.
It’s also crucial to note that most children who take part in clinical trials are already suffering from a serious illness, especially if the trial is considered dangerous.
For instance, when testing cancer treatments, minors almost always take part because they have exhausted other medical options, and therefore the potential benefits of the trial outweigh the risks.
And what about funding?
Aside from ethical barriers, there is also another practical reason why children aren’t always getting the treatments they need.
If we look at the medicines that have been widely studied on minors, such as vaccines or cough medicines, we can see they’re products with a large target market.
That said, most of the serious diseases affecting children are rare diseases, which means it isn’t financially lucrative for companies to invest in treatments to tackle these conditions.
This becomes especially clear when we consider that trials with children cost two to five times more per patient than trials with adults.
As far back as 1999, the EU tried to tackle this problem by offering 10-year market exclusivity for medicines that tackle serious yet uncommon conditions.
Simply put, this means that similar medicines targeting the same illness can’t be placed on the market until a period of ten years has passed.
Whilst this initiative did have a positive impact, the EU has admitted that paediatric research is still being sidelined.
“Where the adult needs or market expectations overlap with paediatric needs, children will benefit directly,” the European Commission said in a report.
Risky for patients and practitioners
When discussing the importance of paediatric clinical trials, you’ll often hear people use the phrase: “Children aren’t just small adults”.
This rallying call for more research reminds us that whilst doctors can use adult studies to predict how a medicine will affect a child, this isn’t a foolproof formula.
A lack of testing on children means that a number of treatments don’t come with dosage guidance for minors, even if anecdotal evidence suggests they’re effective.
This has led to a proliferation of so-called ‘off-label’ prescriptions, which is when a doctor prescribes a drug in an unofficial or unapproved way.
Dr Christoph Male, a paediatric cardiology consultant and associate professor at the Medical University of Vienna, outlines the potential dangers of ‘off-label’ prescriptions.
“Not having appropriate information for the drugs that we use in children is definitely a challenge and a risky situation,” he says. “Risky definitely for our patients but also for us as professionals.”
Although doctors can make dosage calculations based on the weight and age of a young patient, children’s bodies don’t behave in the same way adults’ do.
Critically, a child will metabolise a drug in a very different way to an adult, and doctors must also consider differences in excretion rates, gut permeability, and fat and water distribution.
Dr Male explains that along with other medics in Austria, he is working on a database to help doctors prescribe drugs safely.
“We are running a paediatric formulary that collects all the available information,” he says.
“Most of it is not in any licence information because these drugs have not been licensed for children, but they've been used for many years by paediatricians. [...] There’s long-term experience that’s out there, but it’s not necessarily collected anywhere systematically.”
What’s next?
Building upon measures established in the 2000s, the EU is currently planning a revision of its pharmaceutical legislation.
Whilst progress has been made in paediatric research, long-term solutions are needed to tackle stubborn problems.
Going back to the drawing board, the European Commission (EC) wants to promote innovation by simplifying the PIP process and increasing scientific support given to companies working to address paediatric needs.
The EU revisions are still being finalised, but the EC has also suggested changing the SPC (supplementary protection certificates) system.
SPCs, not to be confused with ‘summary of product characteristics’, give drug companies the right to extend patent exclusivity for new medicines, meaning competitor products can’t enter the market as quickly.
Interestingly, the EU has explored two different approaches here.
On one hand, the bloc has proposed an extension of market exclusivity rights for products that address unmet medical needs.
This would further incentivise companies to develop these medicines.
On the other hand, the EU has also suggested scaling back on some SPC extensions.
Currently, if companies complete a PIP, they are rewarded with a longer period of patent exclusivity.
The European Commission nonetheless says that by scrapping this reward, more products would be able to enter the market earlier.
This could increase the availability of medicines and reduce costs.
Lawmakers are still in the process of debating these revisions, and Austria and Germany even raised the matter at the EU Competitiveness Council in September this year.
Yet whilst lawmakers argue over how to advance children’s medicines, there is a general consensus that paediatric clinical trials are essential.
Research with minors necessarily throws up ethical questions, but conducting fewer trials ultimately means sick children are left with fewer safe treatments.
Many experts suggest that as long as safeguards remain tight, medical testing is not only ethical, but morally essential.