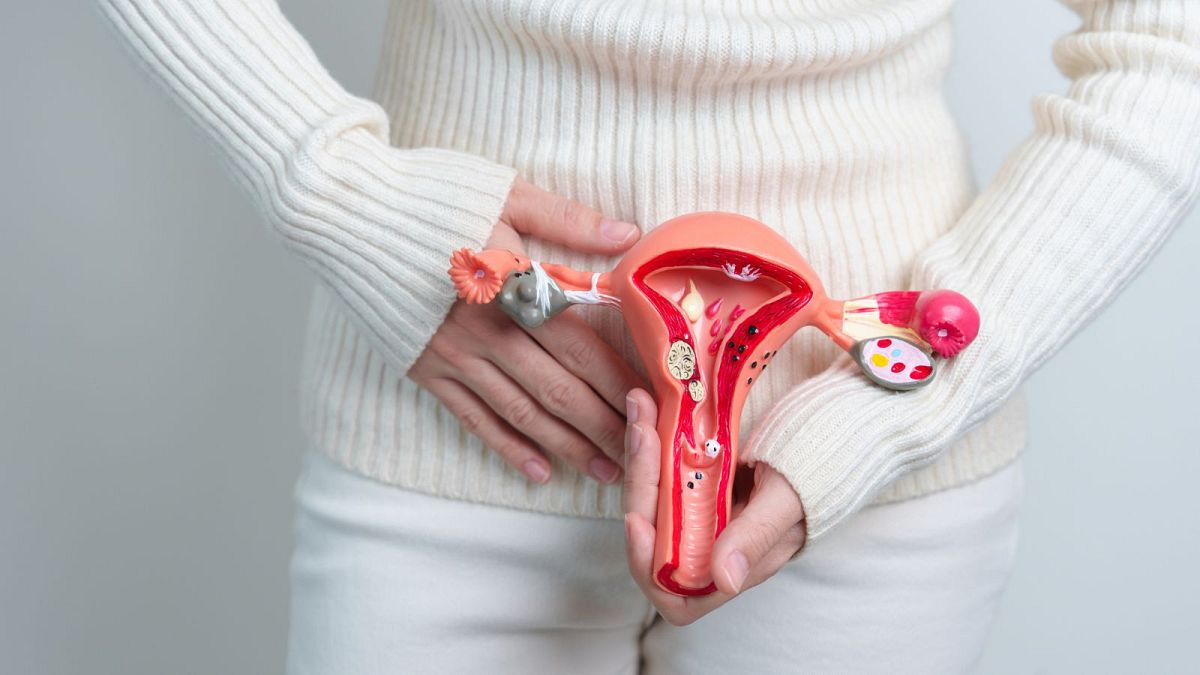
The new drug treatment for uterine cancer, which is already in use in France on an early access scheme, will be used in combination with chemotherapy.
British pharmaceutical company GlaxoSmithKline (GSK) announced on Monday that it has received European approval to market its treatment for endometrial cancer.
Its drug Jemperli is used to treat patients with cancerous cells in their uterus in combination with chemotherapy.
"The European Commission (EC) has granted marketing authorisation for Jemperli (trade name for dostarlimab) in combination with carboplatin-paclitaxel (chemotherapy), for the treatment of adult patients" with newly diagnosed or recurrent advanced endometrial cancer, GSK stated in a press release.
This decision was based on a study demonstrating a positive effect of the treatment on the "progression-free survival" of cancer, meaning the duration during which a patient does not experience worsening of the disease, as well as on "overall survival".
Endometrial cancer, typically affecting postmenopausal women, develops in the inner lining of the uterus, known as the endometrium. It is one of the most common cancers, with over 120,000 new cases annually in Europe.
Although it has a relatively favourable prognosis compared to other female cancers (such as cervical or ovarian cancer), it still leads to numerous fatalities.
Jemperli has been available in France since October under an early access programme, allowing certain patients to receive a treatment that has not yet been formally authorised.