Korea’s capability to control the COVID-19 pandemic has been widely praised. South Korea’s fast turnaround on testing and analysis followed by immediate quarantine and treatment for confirmed cases was regarded as one of the best responses by countries around the world.
 ADVERTISEMENT
ADVERTISEMENT
 ADVERTISEMENT
ADVERTISEMENT
Part of the reason for the success of the South Korean response was due to Seegene’s unique molecular diagnosis technology. Seegene is South Korea based bio-technology company that has pursued and put endless efforts in the area of molecular diagnosis for the past 20 years. In the case of COVID-19, Seegene uses a real-time PCR (polymerase chain reaction) technology that detects the presence of the COVID-19 RNA within a test sample from a patient. Now, real-time PCR is the global standard in molecular diagnostics.
Seegene’s syndromic testing enabled by its real-time PCR test allows detection of multiple target genes to identify the exact cause of the disease from a single tube of reagent.
On top of the accuracy provided by the RT-PCR test, Seegene’s SGDDS (Seegene Digitalized Development System) allowed for a quick response to the fast-spreading COVID-19 pandemic.
Seegene owns its original patented technology DPO™, TOCE™ and MuDT™ technology for multiplex real-time PCR. And such unique technology allows for simultaneous yet precise detection of multiple infections followed by quick procedure of testing.
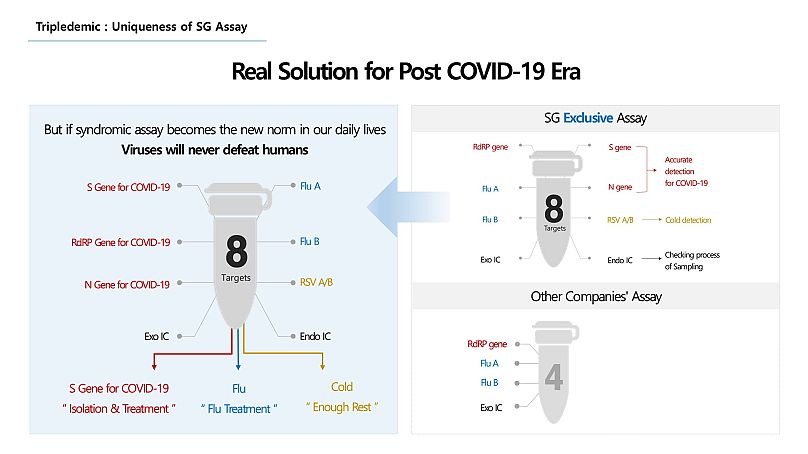
The current technology of testing COVID-19 can only test up to 4 targets with a single tube of reagent, but in September, Seegene introduced another type of testing kit (Allplex™ SARS-CoV-2 /FluA/FluB/RSV Assay) to Europe that can detect 8 different targets to identify COVID-19, seasonal influenza and the common cold in a single tube.
Seegene is currently producing around 50 thousand kits per week, allowing 5 million people to get tested, with 95 percent of Seegene’s diagnostic kits being shipped out to at least 67 countries around the world.
Jong-Yoon Chun, founder and CEO of Seegene believes that with Seegene’s technology, molecular diagnostics can become a part of our daily lives in the near future, and therefore secure a healthier life for all.
Apart from COVID-19 related diagnostic technology, Seegene had already developed molecular diagnosis technology in detecting other diseases, including sexually transmitted infections, gastrointestinal infections and cervical cancer virus.