By Reuters
Share this articleComments
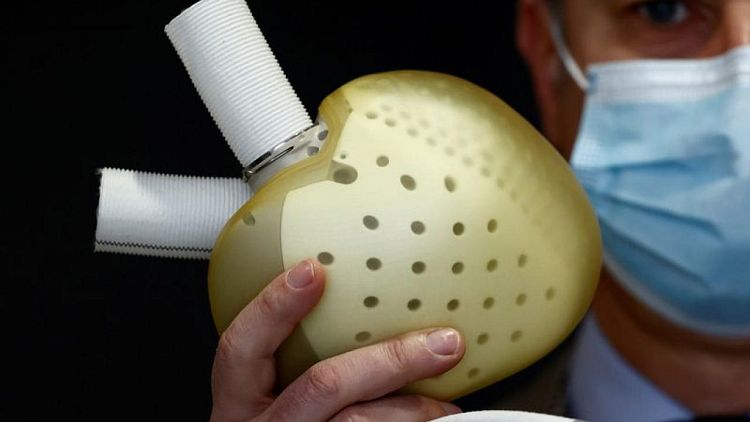
PARIS - French medical devices company CARMAT said it had received the necessary regulatory approvals to resume commercial implants of its Aeson artificial hearts product and would resume sales soon.
"We will be resuming implants very soon and moving forward at a measured pace as we continue building our inventory of implantable prostheses, and ensure all patients are suitably monitored," Chief Executive Stéphane Piat said in a statement.
Share this articleComments