By Amedee Mwarabu
KINSHASA (Reuters) - Democratic Republic of Congo has approved four more experimental treatments against the deadly Ebola virus, the health ministry said as it raced to contain an outbreak in its violence-torn east.
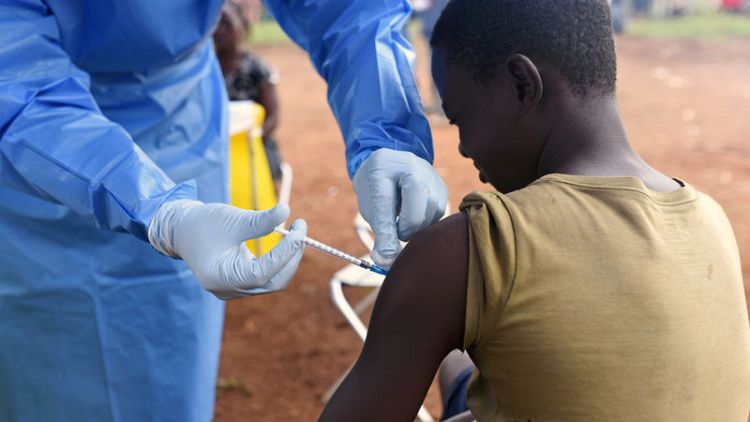
Health authorities last week started administering the U.S.-developed mAb114 treatment to Ebola patients, the first time such a treatment had been used against an active outbreak.
The health ministry said in a daily bulletin late on Tuesday that the 10 patients who received mAb114 since Aug. 11 have experienced a "positive evolution," but the outbreak has continued to grow.
The four additional treatments approved by Congo's ethics committee are Remdesivir, made by U.S.-based Gilead Sciences; ZMapp, an intravenous treatment made by San Diego's Mapp Pharmaceutical; Japanese drug Favipiravir; and Regeneron Pharmaceuticals' Regn3450 – 3471 – 3479.
Remdesivir was administered to its first patient in the town of Beni on Tuesday, who is doing well, the ministry said in its bulletin.
The ministry said on Wednesday that one new case and two new deaths had been confirmed from the haemmorhagic fever, which causes vomiting and severe diarrhoea, bringing the total number of deaths to 61 and confirmed cases to 76 since last month.
Congo, whose heavily forested interior makes its a natural home for Ebola, is at the forefront of a global campaign to combat the virus, which killed more than 11,000 people when it swept through West Africa from 2013-2016.
The Central African country has experienced ten Ebola outbreaks since the virus was discovered in northern Congo in 1976 - more than twice as many as any other country - and 33 people died in a flare-up in the northwest that ended last month.
In addition, a vaccine manufactured by Merck, which proved effective against the earlier outbreak in northwestern Congo, has been administered to 2,179 health workers and contacts of Ebola patients.
In a statement after a cabinet meeting on Wednesday, government spokesman Lambert Mende said that about 5,000 doses of the vaccine remained available.
Insecurity in Congo's eastern borderlands with Uganda has continued to complicate the response, with some contacts of Ebola patients located in so-called "red-zones", which are off limits to emergency responders due to militia activity.
Instead, local health workers in those areas are monitoring the contacts and no Ebola cases have yet been confirmed there.
(Additional reporting by Fiston Mahamba; Writing by Aaron Ross; Editing by Andrew Heavens and Cynthia Osterman)