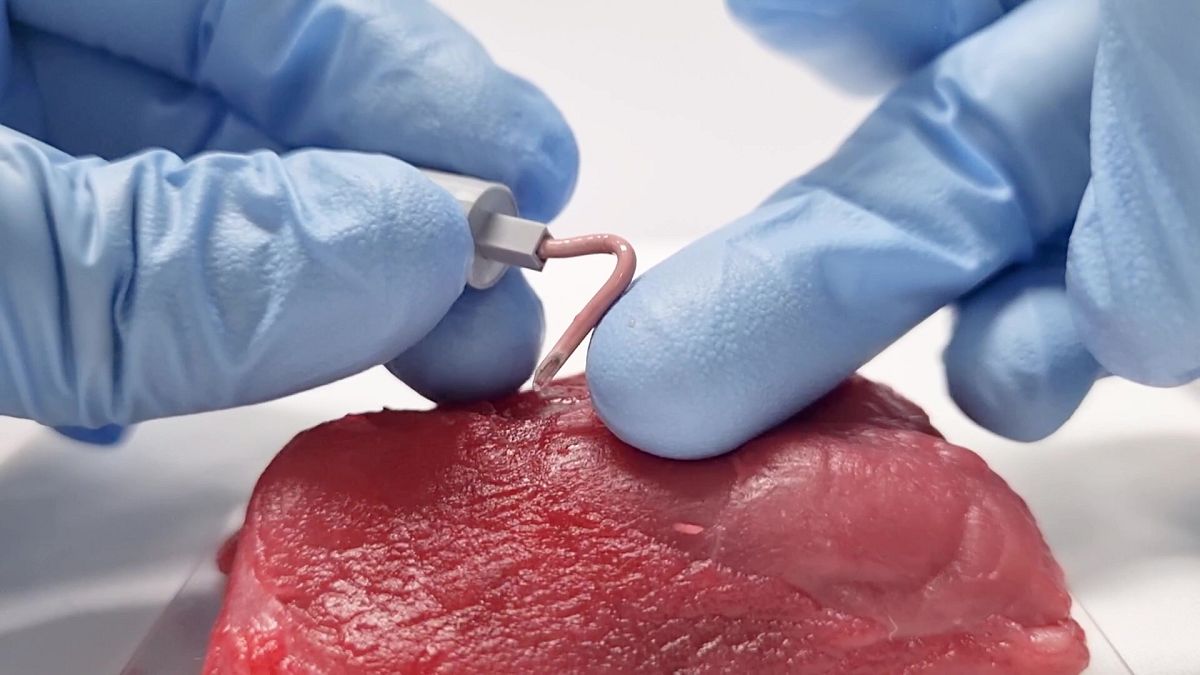
The new IV needle, which is rigid at room temperature like a conventional one, softens when it is inserted and reaches body temperature.
Bioengineers in South Korea have succeeded in developing an intravenous needle (IV) that can bend and flex as it enters the human body.
The softening IV needle is rigid at room temperature, similar to the rigidity of a conventional IV plastic catheter of similar size.
Once it is inserted inside the tissue, it softens within 60 seconds, responding to the body temperature.
Intravenous injection is a commonly used method of directly injecting drugs into blood vessels, which allows medication to rapidly take effect and for continuous drug administration.
But conventional IV needles are made of hard materials such as metal or plastic that can cause damage and inflammation to soft tissues around the injection site.
According to experts, conventional IV needles also often fail before the IVs reach the end of their intended lifespan, primarily due to the movement of the rigid IV in the thin-walled vein.
"Commercially available peripheral intravenous access devices are made up of stainless steel or rigid plastic that does not match with the softness of biological tissues," Karen-Christian Agno, a PhD candidate at the School of Electrical Engineering of Korea Advanced Institute of Science & Technology (KAIST), told Euronews Next.
"This mismatch can cause problems affecting both the patient who will receive the IV injection and the nurse who will give the IV medication. The softening IV needle aims to overcome the current limitations of the commercial rigid IV access devices".
Reducing the risk of damaging tissue
Researchers at KAIST believe that the softening effect of the needle can potentially reduce the risk of damaging the vein of a patient under IV treatment.
Because the needle has a tissue-like softness and is freely deformable, it would allow increased movement and comfort for patients.
The softening needle has potentially benefits not only for patients but also for health workers.
Healthcare workers are often subject to a higher risk of contracting infections, such as Hepatitis B and C, and HIV, especially after the use of IV needles because of their direct contact with blood.
"These concerns can be attributed to the rigidity of the structure of the commercial IV access devices, which the softening IV needle hopes to overcome," said Agno.
It’s made of metal fluid gallium, which has a low melting temperature below the normal body temperature, and good biocompatibility, allowing the needle to soften and mould itself into the shape of vessels.
"The gallium-based needle frame can be made by injection moulding strategy, or by using reusable moulds. The current fabrication does not require any huge equipment or facility, as compared to how conventional medical needles are manufactured," said Agno.
'Widespread' problem: Unethical reuse of IV needles
In 2008, the World Health Organization (WHO) estimated that 40 per cent of the 16 billion injections administered worldwide each year were given with reused syringes and needles without sterilisation.
This led to 1.3 million deaths annually and almost 26 million years of life lost, mainly because of transmission of hepatitis B and C, or HIV.
The researchers behind the study hope that the softening needle can prevent the unethical reuse of IV needles.
Once the needle has been used, it remains soft and non-reusable.
"This means that the needle cannot be reused for another injection. And therefore it can prevent needlestick injuries and reuse of needles," said Agno.
The needle has an ultra-thin temperature sensor inside to help monitor body temperature as changes in body temperature may happen and should be closely monitored when a patient receives an IV medication.
It can also sense unintended leakage of infused fluid in the subcutaneous layers due to a wrongly placed needle.
The softening needle has so far been tested in vein phantoms (devices scientifically designed to resemble human tissue), ex vivo porcine tissue, and mice. As a result, the needle performs similarly in fluid delivery and can even cause less inflammation compared to commercial devices, according to the researchers.
"I personally hope that this device can later be translated for clinical application… Future research work must include further improvement of the encapsulation and packaging of the needle to reduce risk of gallium leakage," Agno said.
"In addition, additional systematic biocompatibility studies, to know the toxicity level of the device for example, are needed to verify the long-term safety of the needle in the vein in relation to the potential gallium leakage," she added.
For more on this story, watch the video in the media player above.