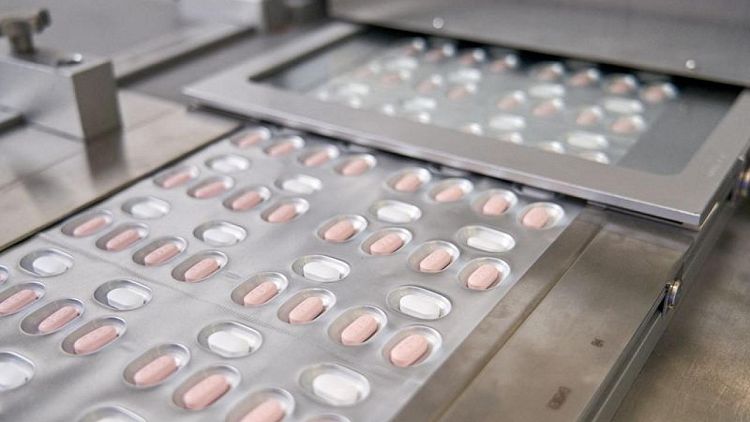
- Pfizer Inc said on Wednesday the U.S. Food and Drug Administration authorized its antiviral COVID-19 pill, making it the first at-home treatment for the coronavirus that is expected to become an important tool in the fight against the fast spreading Omicron variant.
Data from Pfizer's clinical trial showed its two-drug antiviral regimen was 90% effective in preventing hospitalizations and deaths in patients at high risk of severe illness. Recent lab data suggests the drug retains its effectiveness against Omicron.
The agency authorized the oral drug for the treatment of high-risk adult patients and pediatric patients at least 12 years of age with COVID-19 outside of the hospital.
The company said it was ready to start immediate delivery in the U.S. and raised its production projections to 120 million courses of treatment from 80 million in 2022.
The U.S. government's contract for 10 million courses of the Pfizer drug is priced at $530 per course.
The Pfizer pills, taken with the older antiviral drug ritonavir, will be sold under the brand name Paxlovid. The pills are meant to be taken every 12 hours for five days beginning shortly after the onset of symptoms.
Pfizer said it plans to file a new drug application with the FDA in 2022 for potential full regulatory approval.